Science
Groundbreaking Nasal Vaccine Could Revolutionize Respiratory Disease Prevention
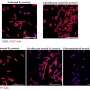
A research team from Trinity College Dublin has introduced a pioneering nasal vaccine that has the potential to transform the prevention of respiratory infections. In a study published in Nature Microbiology, researchers demonstrated that their antibiotic-inactivated Bordetella pertussis (AIBP) vaccine not only prevents severe whooping cough but also limits the transmission of the bacteria—an advancement sought after by vaccine developers globally.
Led by Professor Kingston Mills and Dr. Davoud Jazayeri from the School of Biochemistry and Immunology, this innovative approach utilizes a needle-free mucosal vaccine platform. This platform induces a long-lasting local immune response directly at the site of infection, addressing a critical global need for advanced immunization technologies.
Current vaccines for whooping cough are effective in protecting infants from severe disease; however, they do not prevent the bacteria from colonizing the nose and throat, allowing continued spread within communities. The global resurgence of pertussis, despite high vaccination rates, highlights the urgent demand for improved vaccines.
Professor Mills stated, “We’ve applied our understanding of protective immune pathways to engineer a fundamentally different kind of vaccine. By stimulating immunity where infections begin, at the respiratory mucosa, we can offer stronger protection and potentially interrupt community transmission.”
The Trinity team’s AIBP vaccine distinguishes itself by being delivered intranasally, activating a unique T-cell-driven mucosal immune response. This response effectively protects both the lungs and upper respiratory tract, while minimizing undesirable systemic inflammation. In preclinical studies, the AIBP vaccine provided complete protection against infections in both the lungs and nasal cavity, surpassing the efficacy of current acellular pertussis vaccines.
The findings suggest that AIBP could not only serve as a next-generation pertussis vaccine but also as a versatile platform adaptable to other pathogens, including Staphylococcus aureus, Streptococcus pneumoniae, Mycoplasma pneumoniae, and Mycobacterium tuberculosis.
This significant advancement in vaccine technology stands to reshape how respiratory diseases are approached, offering a hopeful outlook for public health strategies aimed at controlling infections that continue to impact communities worldwide.
For further details, refer to the study by Seyed Davoud Jazayeri et al, titled “Respiratory immunization using antibiotic-inactivated Bordetella pertussis confers T cell-mediated protection against nasal infection in mice,” published in Nature Microbiology in 2025. The DOI for the study is 10.1038/s41564-025-02166-6.
-

 World4 weeks ago
World4 weeks agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World4 weeks ago
World4 weeks agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 Top Stories4 weeks ago
Top Stories4 weeks agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 Science4 weeks ago
Science4 weeks agoTime Crystals Revolutionize Quantum Computing Potential
-

 World4 weeks ago
World4 weeks agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business4 weeks ago
Business4 weeks agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Top Stories4 weeks ago
Top Stories4 weeks agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Lifestyle4 weeks ago
Lifestyle4 weeks agoDiscover Reese Witherspoon’s Chic Dining Room Style for Under $25
-

 Entertainment4 weeks ago
Entertainment4 weeks agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Health4 weeks ago
Health4 weeks agoGavin Newsom Critiques Trump’s Health and National Guard Plans
-

 Business4 weeks ago
Business4 weeks agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts
-

 Science3 weeks ago
Science3 weeks agoRemembering David E. Brest: A Life Dedicated to Nature and Family









