Science
Pfizer’s mRNA Flu Vaccine Shows Promise in Phase 3 Trials
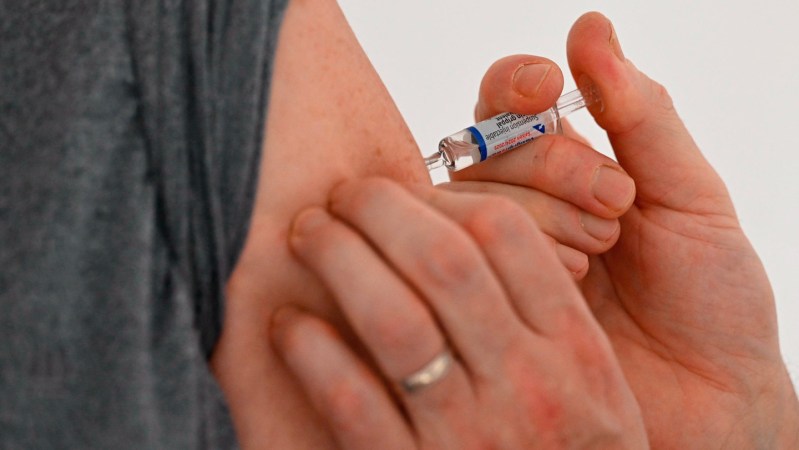
Clinical trials for mRNA flu vaccines are progressing, with promising results from Pfizer’s recent phase 3 trial. Researchers reported in the November 20 issue of the New England Journal of Medicine that the mRNA flu vaccine outperformed traditional vaccines during the 2022–2023 flu season. The trial assessed the effectiveness of Pfizer’s mRNA vaccine by comparing the incidence of flu among participants at least 14 days post-vaccination. The mRNA vaccine demonstrated a relative efficacy of 35 percent over the traditional vaccine, indicating it prevented illness more effectively.
The study involved over 18,000 healthy adults aged 18 to 64 from the United States, South Africa, and the Philippines. The mRNA vaccine specifically targeted hemagglutinin, a vital protein enabling the influenza virus to enter host cells, and included representations of four different flu strains. Each year, a committee from the World Health Organization (WHO) determines which strains should be included in seasonal vaccines.
In June, Moderna also reported phase 3 trial results for its mRNA flu vaccine, aimed at adults aged 50 and older. This trial, which involved nearly 41,000 participants across 11 countries, showed a relative efficacy of around 27 percent compared to traditional flu vaccines. Given that individuals aged 65 and older face a higher risk of severe flu complications, an effective mRNA flu vaccine could significantly enhance preventive measures against the influenza virus.
One key advantage of the mRNA platform is its expedited production process. Traditional vaccines require significant lead time, as the WHO’s recommendations for vaccine composition must be established early enough to accommodate the typically six-month manufacturing period. This delay can hinder the ability to respond to late-emerging flu strains. For instance, during this year’s Northern Hemisphere flu season, a variant that gained traction during the Southern Hemisphere season has dominated flu samples in England and Japan. Unfortunately, this variant surfaced too late to be incorporated into the current season’s vaccine formulation.
As clinical trials advance, the potential for mRNA vaccines to reshape the landscape of influenza prevention becomes increasingly apparent. The ability to adapt swiftly to the evolving nature of the virus could provide a new, valuable tool in combating seasonal flu outbreaks and enhancing public health measures globally.
-

 Top Stories2 months ago
Top Stories2 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 World2 months ago
World2 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-
 Science2 weeks ago
Science2 weeks agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 World2 months ago
World2 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-
 World2 months ago
World2 months agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business2 months ago
Business2 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Top Stories2 months ago
Top Stories2 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment2 months ago
Entertainment2 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Business2 months ago
Business2 months agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts
-

 Health2 months ago
Health2 months agoGavin Newsom Critiques Trump’s Health and National Guard Plans
-

 Lifestyle2 months ago
Lifestyle2 months agoDiscover Reese Witherspoon’s Chic Dining Room Style for Under $25








