Health
Armata Pharmaceuticals Unveils Promising Phase 2a Results for AP-SA02

Armata Pharmaceuticals, Inc. has reported positive outcomes from its Phase 2a diSArm study, evaluating the bacteriophage cocktail AP-SA02 as a potential treatment for complicated Staphylococcus aureus (S. aureus) bacteremia. The results were shared during a late-breaking oral presentation at IDWeek 2025, held from October 19 to 22 in Atlanta, Georgia.
The study, titled “A Phase 2a Randomized, Double-Blind, Controlled Trial of the Efficacy and Safety of an Intravenous (IV) Bacteriophage Cocktail (AP-SA02) vs. Placebo in Combination with Best Available Antibiotic Therapy (BAT) in Patients with Complicated Staphylococcus aureus Bacteremia,” showcased findings presented by Dr. Loren G. Miller, M.D., M.P.H. He is a Professor of Medicine at the David Geffen School of Medicine at UCLA and Chief of the Division of Infectious Diseases at Harbor-UCLA Medical Center.
Dr. Miller emphasized the significance of the results, stating, “The results of the diSArm study confirm, for the first time in a randomized clinical trial, the efficacy of intravenous phage therapy for S. aureus bacteremia.” He expressed confidence in the findings, which suggest a strong rationale for advancing to a Phase 3 superiority study. If successful, this could pave the way for integrating AP-SA02 into clinical practice for managing S. aureus bacteremia, a severe and potentially fatal infection.
Dr. Deborah Birx, Chief Executive Officer of Armata, also highlighted the milestone achieved with the diSArm study. “The positive results from the diSArm study represent another significant achievement for Armata as we aim to advance AP-SA02 into a pivotal trial,” she noted. Dr. Birx acknowledged the contributions of Dr. Miller and his team, along with the support from partners, including the U.S. Department of Defense and Innoviva.
The Phase 2a study enrolled a total of 42 patients, with 29 receiving AP-SA02 in combination with BAT and 13 receiving a placebo. The study found that approximately 38% of participants in both groups were infected with methicillin-resistant S. aureus (MRSA). Clinical responses were evaluated on day 12 and at the end of the study, four weeks after treatment.
In the intent-to-treat population, the AP-SA02 group demonstrated a higher clinical response rate at day 12, with 88% achieving a positive outcome compared to 58% in the placebo group, as assessed by blinded investigators. Furthermore, no patients in the AP-SA02 group experienced non-response or relapse at either of the assessed time points. In contrast, the placebo group reported non-response/relapse rates of 25% at both evaluations.
Patients receiving AP-SA02 also showed quicker normalization of C-reactive protein levels, faster negative blood culture results, and reduced hospitalization times. The study reported no serious adverse events related to the investigational drug, indicating a favorable safety profile. Treatment-emergent adverse events occurred in only 6% of the AP-SA02 group compared to none in the placebo group.
New findings from the study indicated that the genomic variants present in the AP-SA02 formulation may allow for rapid, strain-specific responses to individual patient isolates of S. aureus. This adaptability could be crucial for optimizing the efficacy of phage therapy.
In conclusion, the results from the diSArm study suggest that AP-SA02, when combined with BAT, offers a higher and earlier cure rate for patients with complicated S. aureus bacteremia. The absence of non-responses or relapses in the treatment group, combined with the observed safety profile, supports the advancement into a pivotal Phase 3 trial planned for 2026, pending feedback from the U.S. Food and Drug Administration (FDA).
The development of AP-SA02 represents a significant step forward in Armata’s efforts to establish effective treatments for patients suffering from complicated S. aureus bacteremia. This innovative approach to phage therapy may soon become a vital option in combating antibiotic-resistant bacterial infections.
IDWeek 2025 is a collaborative meeting involving multiple professional organizations, including the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America, focusing on the latest advancements in infectious disease science and treatment. For more information, visit www.idweek.org.
-
 Science4 weeks ago
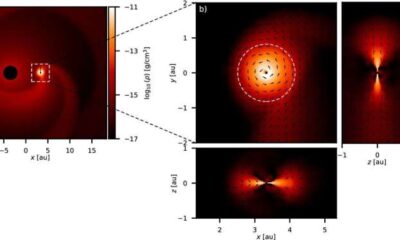
Science4 weeks agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 Top Stories2 months ago
Top Stories2 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 Politics2 months ago
Politics2 months agoSEVENTEEN’s Mingyu Faces Backlash Over Alcohol Incident at Concert
-

 World2 months ago
World2 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World2 months ago
World2 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-
 World2 months ago
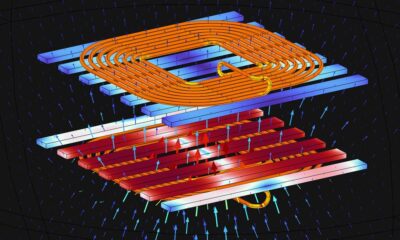
World2 months agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business2 months ago
Business2 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Top Stories2 months ago
Top Stories2 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment2 months ago
Entertainment2 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Health2 months ago
Health2 months agoGavin Newsom Critiques Trump’s Health and National Guard Plans
-

 Business2 months ago
Business2 months agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts









