Health
Botulism Outbreak Linked to ByHeart Formula Affects 51 Infants

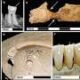
An outbreak of infant botulism linked to ByHeart formula has resulted in hospitalization for 51 babies across 19 states, according to the Food and Drug Administration (FDA). This serious illness can lead to paralysis and, in some cases, death. The FDA’s announcement on December 10, 2025, revealed an increase from the previous report of 39 cases affecting 18 states earlier this month.
The outbreak’s timeline stretches back to illnesses that began between August 9 and November 19, 2025, with confirmed or suspected cases linked to ByHeart powdered infant formula. The affected infants are aged between two weeks and five months. As of now, no fatalities have been reported. The states involved include Arizona, California, Idaho, Illinois, Kentucky, Massachusetts, Maine, Michigan, Minnesota, North Carolina, New Jersey, Ohio, Oregon, Pennsylvania, Rhode Island, Texas, Virginia, Washington, and Wisconsin. Notably, Ohio was the latest state added to this alarming list.
The FDA indicated that it has not received reports of recalled formula being present on store shelves since November 26, 2025. In light of the outbreak, all ByHeart infant formula products have been recalled. The FDA emphasized that these products should not be available for sale in stores or online. This includes all formula cans and single-serve “anywhere pack” sticks.
Despite the ongoing recall, some consumers have reported finding ByHeart products on the shelves of major retailers, including Walmart, Target, and Kroger. ByHeart, which represents approximately 1% of the U.S. infant formula market, previously sold about 200,000 cans each month.
Testing is currently underway involving ByHeart, the FDA, the Centers for Disease Control and Prevention (CDC), and state health officials, with results anticipated in the coming weeks. ByHeart has urged parents and caregivers who possess the formula to cease usage immediately and dispose of the product. The FDA has specifically recalled certain lots of ByHeart Whole Nutrition Infant Formula, detailed on their website.
California health officials confirmed that a sample from an open can of ByHeart baby formula, which was fed to an infant who subsequently fell ill, tested positive for the bacteria responsible for botulism. Dr. Erica Pan, the state health officer, explained the testing process involves injecting mice with the cultured bacterium and observing them for signs of sickness over a period of up to four days. “These mice got sick really quickly,” Pan noted.
As of now, investigators have not identified any other infant formula brands or sources of exposure related to this outbreak. ByHeart released a statement on November 9, indicating they are conducting testing of the recalled batches with a third-party independent laboratory. The company has acted swiftly to remove any potential risk, recalling the relevant batches of formula as identified by the FDA.
Botulism is a rare but serious disease caused by a type of bacteria that produces toxins in the intestines. Symptoms can take weeks to manifest and may include poor feeding, loss of head control, drooping eyelids, and a flat facial expression. Infants are particularly susceptible due to their underdeveloped gut microbiomes, which cannot prevent the spores from germinating and producing toxins.
The only known treatment for infant botulism is BabyBIG, an intravenous medication made from the pooled blood plasma of adults immunized against botulism. The CDC confirmed that all affected children in this outbreak have received this treatment, which is priced at approximately $69,300 per vial.
Experts have suggested that there is minimal risk of a nationwide infant formula shortage due to ByHeart’s relatively small market share. This situation differs significantly from the formula crisis in late 2021 and 2022 when several infants were sickened after consuming formula produced by Abbott Nutrition, leading to a nationwide shortage.
In 2022, ByHeart had previously recalled five batches of infant formula after a sample from their packaging plant tested positive for a different bacterium, cronobacter sakazakii. Following that incident, the FDA issued a warning letter detailing areas that required corrective actions.
As health officials continue to monitor the situation, parents are advised to stay informed and adhere to safety recommendations regarding infant formula use.
-

 Science4 weeks ago
Science4 weeks agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 Top Stories2 months ago
Top Stories2 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 Politics2 months ago
Politics2 months agoSEVENTEEN’s Mingyu Faces Backlash Over Alcohol Incident at Concert
-

 World2 months ago
World2 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World2 months ago
World2 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 World2 months ago
World2 months agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business2 months ago
Business2 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Top Stories2 months ago
Top Stories2 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment2 months ago
Entertainment2 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Business2 months ago
Business2 months agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts
-

 Health2 months ago
Health2 months agoGavin Newsom Critiques Trump’s Health and National Guard Plans