Health
New Research Reveals Microglia’s Role in Alzheimer’s Lipid Imbalance
Research from **UT Health San Antonio** in collaboration with the **University of California at Irvine** has unveiled significant insights into the role of brain immune cells in Alzheimer’s disease. The study identifies how targeting microglia, the brain’s immune cells, could help restore lipid imbalances associated with this degenerative condition. These findings highlight a critical aspect of Alzheimer’s that has been overlooked: the disruption of brain lipids alongside the more commonly studied amyloid-beta and tau proteins.
More than a century ago, **Alois Alzheimer** observed unusual fat changes in the brain, which he referred to as “lipoid granules.” Since then, most research has concentrated on amyloid and tau, while the impact of lipid abnormalities has not been thoroughly explored. The recent study emphasizes that lipid imbalances significantly influence the buildup of amyloid proteins and are linked to genetic factors that heighten Alzheimer’s risk.
Microglia’s Dual Role in Alzheimer’s Progression
According to **Juan Pablo Palavicini, Ph.D.**, co-lead of the study and assistant professor at UT San Antonio’s Long School of Medicine, “The brain is a unique organ. Unlike most other organs, which are rich in protein, more than half of the brain’s dry weight is made up of different kinds of lipids.” The research, published in **Nature Communications** in 2025, reveals that microglia can either maintain lipid balance or exacerbate the disease, depending on how they are manipulated.
Using a mouse model of Alzheimer’s, the research team tested two methods to deplete microglia: one involved a drug that nearly eliminated all microglia, while the other utilized genetically modified mice that lack these immune cells. This approach allowed the scientists to discern the specific effects of microglia from those of other brain cells.
Palavicini noted, “We wanted to understand which cells are driving these lipid changes.” The research team compared outcomes from the mouse studies with post-mortem brain samples from both Alzheimer’s patients and healthy individuals. They found that amyloid buildup significantly altered brain lipid patterns, revealing the importance of certain lipids in the disease’s progression.
Key Lipids and Their Roles
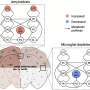
Two lipid groups emerged as particularly significant: **lysophospholipids (LPC and LPE)**, which are associated with inflammation and oxidative stress, and **bis(monoacylglycero)phosphate (BMP)**, a lipid crucial for regulating cell recycling processes. The researchers discovered that a variant of BMP containing **arachidonic acid (AA-BMP)** accumulated near amyloid plaques. Notably, long-term microglia depletion prevented the buildup of AA-BMP, indicating that these immune cells drive these critical changes.
“BMP is still not well understood, especially in the brain,” Palavicini explained. “It forms substructures in lysosomes that attract proteins to break down damaged lipids. Without microglia, AA-BMP levels drop, which can interfere with the brain’s cleanup processes.”
The study also highlighted the role of **progranulin**, a protein produced by both microglia and neurons, as a vital regulator of lipid metabolism. Elevated levels of progranulin in Alzheimer’s conditions align closely with increased AA-BMP accumulation. The removal of microglia resulted in decreased levels of both progranulin and AA-BMP near amyloid plaques, suggesting that microglial progranulin plays a crucial role in maintaining lipid balance.
Palavicini remarked, “In the Alzheimer’s brain, rather than lowering BMP, it may be important to maintain or support its levels. Progranulin helps maintain this lipid and protects neurons.” This insight suggests that therapies aimed at enhancing progranulin levels could potentially restore lipid balance and promote brain health.
The research also revealed that not all lipid levels are influenced by microglia. Increases in LPC and LPE were mainly driven by astrocytes and neurons. LPC accumulation was linked to astrocyte activation, while LPE rises correlated with oxidative stress and diminished antioxidant defenses. This distinction is essential for understanding which cell types to target for future therapies.
Implications for Alzheimer’s Treatment
This groundbreaking research indicates that Alzheimer’s disease involves more than just amyloid plaques and tau tangles; it also encompasses disrupted lipid balance, with microglia, astrocytes, and neurons each playing distinct roles. Microglia are shown to maintain protective lipids like BMP and support myelin, while other cell types contribute to the accumulation of inflammatory lipids.
“Understanding which cells regulate which lipids opens the door to more precise therapies,” Palavicini stated. “By targeting lipid balance alongside amyloid and tau, we can develop better strategies to protect neurons and potentially slow or prevent Alzheimer’s disease.”
The findings of this study promise to reshape the approach to Alzheimer’s research and treatment, highlighting the necessity of addressing lipid imbalances as a critical component of effective therapeutic strategies.
-

 Top Stories2 months ago
Top Stories2 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 World2 months ago
World2 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 Science2 weeks ago
Science2 weeks agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 World2 months ago
World2 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 World2 months ago
World2 months agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business2 months ago
Business2 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Top Stories2 months ago
Top Stories2 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment2 months ago
Entertainment2 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Business2 months ago
Business2 months agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts
-

 Health2 months ago
Health2 months agoGavin Newsom Critiques Trump’s Health and National Guard Plans
-

 Lifestyle2 months ago
Lifestyle2 months agoDiscover Reese Witherspoon’s Chic Dining Room Style for Under $25









