Health
SS Innovations Seeks FDA Approval for Mantra Robotic Surgery System

SS Innovations, a company focused on making robotic surgery more accessible, has officially submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its SSi Mantra surgical robotic system. This system is designed for a variety of specialty procedures, including general, urological, colorectal, gynecological, and cardiac surgeries. The submission marks a significant step in the company’s efforts to introduce its advanced surgical technology to the U.S. market.
Dr. Sudhir Srivastava, Chairman and CEO of SS Innovations, expressed optimism about the submission. He stated, “Our submission of a 510(k) premarket notification to the FDA marks an important milestone in our strategic plan to introduce the Company’s advanced, cost-efficient SSi Mantra surgical robotic system to the U.S. market.” Dr. Srivastava emphasized the affordability and performance of the SSi Mantra, noting its potential impact on hospitals and surgeons, particularly those serving underserved communities.
Regulatory Pathway and Approval Timeline
Following a pre-submission meeting and discussions with the FDA, SS Innovations opted for the 510(k) route instead of a De Novo request. This decision was based on the expectation of faster and more cost-effective approval processes. The FDA aims to complete reviews of 510(k) submissions within 90 days of receipt, although various factors, including a 15-day acceptance review and the need for additional information, could extend this timeline.
In addition to pursuing FDA approval, SS Innovations is also working towards obtaining a CE marking certification in the European Union. The company anticipates achieving this certification in the first half of 2026.
Global Presence and Surgical Impact
As of November 30, 2025, the SSi Mantra system has established a cumulative installed base of 138 systems across eight countries where it has received regulatory approval. To date, 137 hospitals have integrated the SSi Mantra into their surgical operations, leading to the successful completion of over 7,300 surgical procedures. This includes 88 telesurgeries and 390 cardiac procedures, demonstrating the system’s versatility and effectiveness.
The progress of SS Innovations reflects a growing trend in the healthcare industry, where innovative technologies aim to improve surgical outcomes while also addressing cost barriers. With the potential approval of the SSi Mantra, the company could play a crucial role in expanding access to advanced surgical solutions in the U.S. and beyond.
-
 Science4 weeks ago
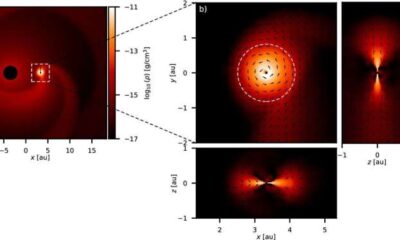
Science4 weeks agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 Top Stories2 months ago
Top Stories2 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 Politics2 months ago
Politics2 months agoSEVENTEEN’s Mingyu Faces Backlash Over Alcohol Incident at Concert
-

 World2 months ago
World2 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World2 months ago
World2 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-
 World2 months ago
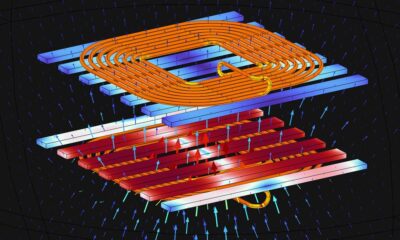
World2 months agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business2 months ago
Business2 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Top Stories2 months ago
Top Stories2 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment2 months ago
Entertainment2 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Business2 months ago
Business2 months agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts
-

 Health2 months ago
Health2 months agoGavin Newsom Critiques Trump’s Health and National Guard Plans









