Health
New Gene Discovery Methods Transform Understanding of Disease Biology

A recent study published in the journal Nature reveals that two primary methods for gene discovery offer complementary insights into disease biology. Conducted by a team from NYU Langone Health, Stanford University, UC San Francisco, and the University of Tokyo, the research highlights how these distinct approaches identify different sets of genes, which could significantly impact drug development.
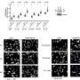
The study focuses on the human genome, which comprises thousands of genes responsible for producing proteins and regulatory DNA that dictates gene expression. By examining genome-wide association studies (GWAS) and burden tests across 209 traits from the UK Biobank, the researchers uncovered essential differences in how these methods pinpoint genetic variations linked to diseases.
Distinct Methods Reveal Unique Insights
GWAS typically examines common variants across the genome, encompassing both genes and regulatory regions. In contrast, burden tests concentrate on rare variants that alter protein function. The findings indicate that burden tests tend to identify genes that predominantly influence specific diseases, while GWAS can reveal genes that impact multiple diseases and biological processes.
According to Hakhamanesh Mostafavi, Ph.D., co-senior author and assistant professor at the NYU Grossman School of Medicine, “Our study explains why the methods produce different results and why both are biologically important.” This clarification is crucial for understanding how genetic findings can be utilized in applications like drug development.
Despite the utility of GWAS in identifying disease-associated genes, the method often implicates hundreds of genes, complicating the task of determining which are genuinely significant. The advent of larger biobanks has enhanced the power of burden tests, providing a clearer picture of the fewer, more interpretable genes related to specific diseases.
Understanding Gene Variability and Its Implications
The researchers discovered that the disparity in results between GWAS and burden tests stems from the nature of the genes themselves. Some genes have a narrow influence, affecting a single trait, while others impact multiple traits simultaneously. Variants that significantly disrupt these multi-trait genes are typically pruned by evolution, as they often hinder survival or reproduction, making them rarer and challenging for burden tests to detect.
Conversely, GWAS can identify these genes because regulatory DNA variants often alter gene activity in less extreme ways, allowing them to persist through evolutionary pressures. The study emphasizes two key features for prioritizing genes related to disease risk: “importance,” which reflects the degree to which a gene impacts disease when disrupted, and “specificity,” indicating whether a gene primarily affects one disease or multiple traits.
Additionally, the study reveals that the p-values derived from GWAS and burden tests serve as inadequate indicators of a gene’s importance. Mostafavi notes, “Our results do not mean that GWAS and burden tests lack useful information; they just have not been interpreted in this way before.”
Looking ahead, the research team aims to develop innovative methods to better prioritize genes based on their significance in disease contexts. By merging results from both GWAS and burden tests with expanding experimental data about gene functions within cells, they anticipate leveraging machine learning techniques to identify critical patterns and refine estimates of gene importance.
The implications of this research are profound, as highlighted by co-senior author Jeffrey Spence, Ph.D., from UC San Francisco. He asserts, “This would be revolutionary because it would let us leverage all of the cell-level experimental data to learn about human-level traits, identify the most important disease genes, and streamline drug development.”
As researchers continue to explore the complex relationship between genes and diseases, this study offers vital insights that could reshape the landscape of genetic research and its applications in medicine. The team’s ongoing work will likely pave the way for more effective therapeutic targets and improved understanding of genetic contributions to health and disease.
-

 World3 weeks ago
World3 weeks agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World3 weeks ago
World3 weeks agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 Science3 weeks ago
Science3 weeks agoTime Crystals Revolutionize Quantum Computing Potential
-

 World3 weeks ago
World3 weeks agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Top Stories3 weeks ago
Top Stories3 weeks agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 Top Stories3 weeks ago
Top Stories3 weeks agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment3 weeks ago
Entertainment3 weeks agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoDiscover Reese Witherspoon’s Chic Dining Room Style for Under $25
-

 Health3 weeks ago
Health3 weeks agoGavin Newsom Critiques Trump’s Health and National Guard Plans
-

 Politics1 week ago
Politics1 week agoLanguage Evolution: New Words Spark Confusion in Communication
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoLia Thomas Honored with ‘Voice of Inspiration’ Award at Dodgers Event
-

 Entertainment3 weeks ago
Entertainment3 weeks agoFast & Furious Coaster Hits the Track at Universal Studios