Top Stories
Structure Therapeutics Shares Soar 102% on Promising Obesity Pill Data

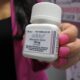
UPDATE: Structure Therapeutics (NASDAQ: GPCR) has just announced a staggering 102% surge in its share price, following the release of groundbreaking mid-stage clinical data for its oral obesity treatment, aleniglipron. This remarkable leap comes on December 8, 2025, as investors react swiftly to the promising results, doubling the stock price from $34.56 to $69.98.
The Phase II ACCESS clinical trials revealed that patients treated with aleniglipron experienced a 11.3% mean weight loss after 36 weeks, showcasing its potential as a formidable competitor to established obesity treatments from giants like Eli Lilly and Novo Nordisk. The treatment, which is designed to target weight-related co-morbidities, could pave the way for a new era in obesity management.
This news comes as Eli Lilly (NYSE: LLY) and Novo Nordisk (Nasdaq Copenhagen: NOVO-B) race to launch their own oral GLP-1 receptor agonists. Novo Nordisk’s Wegovy® and Lilly’s orfoglipron are both under FDA review, with approvals anticipated in the near future. Novo recently shared data indicating a 13.6% weight loss for participants taking Wegovy, while Lilly’s orfoglipron showed an average weight reduction of 10.5% (22.9 lbs).
Structure Therapeutics’ CEO, Raymond Stevens, PhD, expressed optimism, calling the results “potentially best-in-class for oral small molecule GLP-1s.” The data reveal aleniglipron’s efficacy, with participants in the ACCESS II trial achieving a remarkable 15.3% weight loss on a higher dose.
Despite the excitement, analysts highlight some caution. An expert from William Blair, Andy T. Hsieh, PhD, suggests it’s too early to label aleniglipron as best-in-class but acknowledges its significant market potential. Hsieh raised his sales forecast for aleniglipron in the U.S. and Europe by 53%, projecting peak sales of $5.8 billion.
However, the ACCESS trials reported a higher incidence of vomiting among participants compared to Lilly’s trials for orfoglipron, which could present challenges. Structure aims to mitigate these side effects with a lower starting dose in further studies.
The FDA has granted two Priority Vouchers to orfoglipron and Wegovy, expediting their review processes. This could lead to a competitive landscape in the obesity treatment market, particularly as Structure prepares for a Type B End-of-Phase II meeting with the FDA in the first half of next year.
Investors are not just excited about aleniglipron’s data. The market buzz suggests that Structure could be a takeover target, akin to recent bidding wars in the obesity drug sector. Analysts are raising their price targets, reflecting a growing belief in Structure’s potential.
As the obesity treatment field heats up, Structure’s impressive results are positioning it as a key player. With FDA meetings on the horizon and further trials planned, all eyes will be on how aleniglipron performs in the competitive landscape.
This rapidly evolving situation underscores the urgent need for effective obesity treatments, as millions around the globe grapple with weight-related health issues. The market will be watching closely as these developments unfold.
-

 Science1 month ago
Science1 month agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 Politics2 months ago
Politics2 months agoSEVENTEEN’s Mingyu Faces Backlash Over Alcohol Incident at Concert
-

 Top Stories2 months ago
Top Stories2 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 World2 months ago
World2 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World2 months ago
World2 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 World2 months ago
World2 months agoElectrification Challenges Demand Advanced Multiphysics Modeling
-

 Business2 months ago
Business2 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Top Stories2 months ago
Top Stories2 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Entertainment2 months ago
Entertainment2 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Business2 months ago
Business2 months agoUS Government Denies Coal Lease Bid, Impacting Industry Revival Efforts
-

 Health2 months ago
Health2 months agoGavin Newsom Critiques Trump’s Health and National Guard Plans