Science
Researchers Unveil Mechanism Behind Cell Communication Using CRISPR
A research team from the University of Barcelona has identified a crucial molecular mechanism involved in how cells communicate, specifically through extracellular vesicles (EVs). These small particles, which have significant therapeutic potential, play a vital role in cellular information exchange. The findings, published on November 28, 2025, in the Journal of Extracellular Vesicles, highlight the importance of the Commander protein complex, known previously for its involvement in membrane recycling.
Led by Professor Albert Lu from the Faculty of Medicine and Health Sciences at the University of Barcelona and the CELLEX Biomedical Research Center (IDIBAPS-UB), the study involved collaboration with María Yáñez-Mó from the Severo Ochoa Center for Molecular Biology (CSIC-UAM) and Carles Enrich, also from IDIBAPS-UB. According to Lu, “Understanding how receptor cells capture and process extracellular vesicles is essential to understanding how our body communicates at the molecular level.”
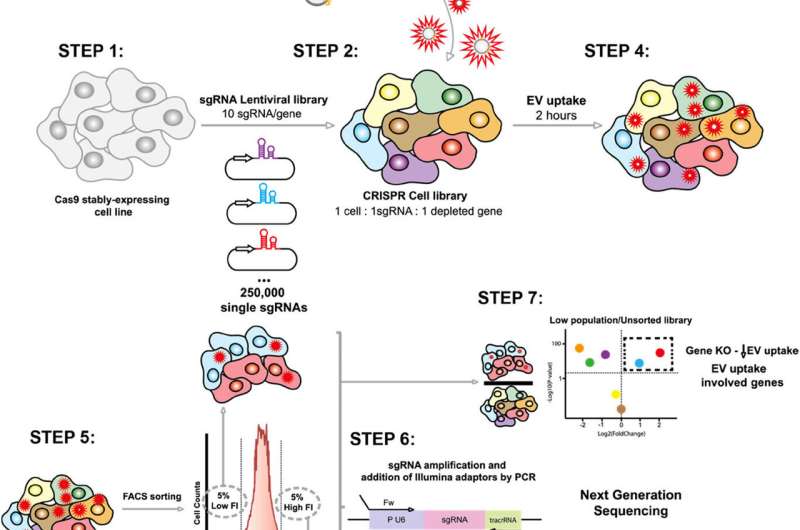
The research utilized an innovative approach combining CRISPR-Cas9 technology and massive genomic screening to dissect the molecular mechanisms that govern the uptake and internalization of EVs. This powerful tool enables researchers to deactivate each of the more than 20,000 human genes individually, allowing for a comprehensive analysis of their roles in the process.
Cells were genetically modified to deactivate specific genes, after which they were exposed to EVs labeled with a fluorescent dye. Using flow cytometry, the researchers measured the extent of vesicle capture by the modified cells. Subsequently, fluorescence-activated cell sorting (FACS) helped isolate cells with varying uptake capacities, and mass sequencing was used to identify the deactivated genes.
“This systematic and unbiased approach allows us to discover new regulators without relying on prior hypotheses, unlike traditional techniques that focus on specific candidates,” Lu stated. The results indicate that the Commander endosomal recycling complex, comprising various proteins, serves as a fundamental regulator of vesicle uptake. The study’s findings across different human cell lines suggest that the mechanism is potentially universal, although its effectiveness may vary by cell type or physiological context.
Understanding this communication process has significant therapeutic implications. The ability of EVs to cross membranes and target specific tissues positions them as promising natural vehicles for drug delivery or therapeutic molecules. Lu emphasized, “Understanding how their entry, intracellular trafficking, and delivery of their molecular cargo are regulated opens the door to designing EVs with controlled directionality, improving their efficacy in regenerative, oncological, or anti-inflammatory therapies.”
The research team is actively pursuing further investigations to gain a more nuanced understanding of the Commander complex’s role in controlling vesicle uptake and their intracellular fate. They aim to determine if this mechanism is conserved across various cell types or tissues. Additionally, they are exploring whether dysfunctions in this complex could contribute to altered cell communication in pathological conditions such as cancer or neurodegenerative diseases.
“In the long term, the goal is to be able to manipulate this pathway to modulate communication between cells and enhance the use of EVs as therapeutic and diagnostic tools,” Lu concluded.
This groundbreaking research not only advances the understanding of cellular communication but also paves the way for future innovations in therapeutic applications using extracellular vesicles.
-

 Science3 months ago
Science3 months agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 Science3 months ago
Science3 months agoDoctoral Candidate Trivanni Yadav Advances Battery Research at UTulsa
-

 World4 months ago
World4 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 Top Stories4 months ago
Top Stories4 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 World4 months ago
World4 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 Sports3 months ago
Sports3 months agoNASCAR Faces Fan Backlash as Steve Phelps’ Texts Surface
-

 Business4 months ago
Business4 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Top Stories4 months ago
Top Stories4 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Politics4 months ago
Politics4 months agoSEVENTEEN’s Mingyu Faces Backlash Over Alcohol Incident at Concert
-

 Science4 months ago
Science4 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Entertainment4 months ago
Entertainment4 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Science4 months ago
Science4 months agoRemembering David E. Brest: A Life Dedicated to Nature and Family