Health
Vertex Faces Delays in Gene Therapy for Sickle Cell Disease
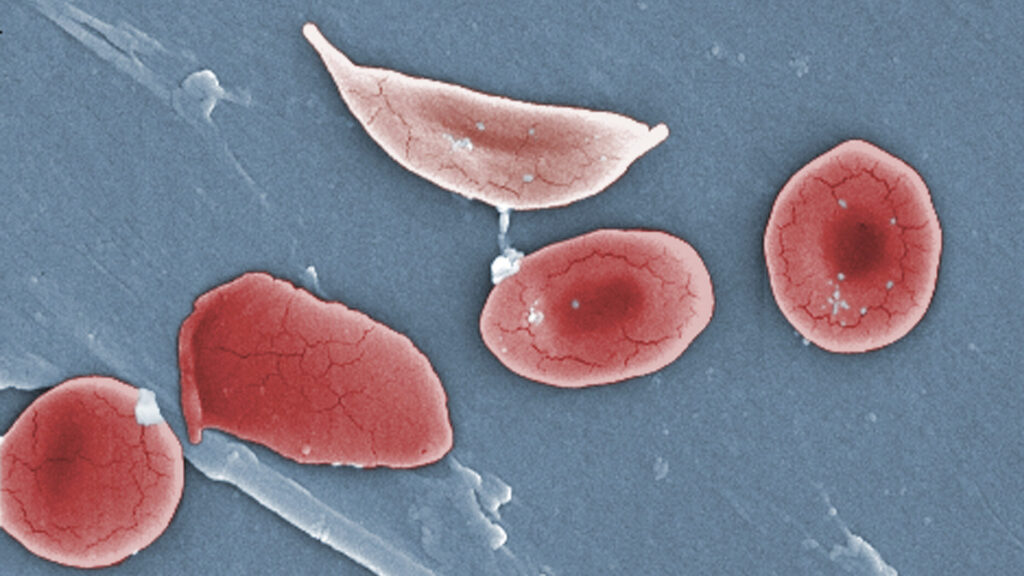
Vertex Pharmaceuticals is encountering significant delays in the rollout of its gene-editing therapy, Casgevy, for treating sickle cell disease. Although the therapy received approval over two years ago in 2021, only about 60 patients across the United States, Middle East, and Europe have been treated with this innovative approach. This sluggish progress has taken even industry specialists by surprise, particularly concerning the challenges in collecting enough cells necessary for the treatment.
According to experts from four specialized sickle cell treatment centers, the primary hurdle contributing to this slow adoption is the difficulty in gathering sufficient patient cells. This step is essential for producing the customized therapy that Casgevy requires. The situation is particularly pressing for Vertex as it seeks to solidify its position in the marketplace amidst increasing competition from rival therapies.
Vertex executives had previously indicated that the path to widespread availability for Casgevy might be slow, but the current pace has exceeded expectations. The company is working to address the cell collection issues, which are critical for patient access to the therapy. As the market landscape evolves, Vertex also faces the impending entry of a major competitor in the upcoming year, heightening the urgency to resolve these logistical challenges.
The implications of these delays extend beyond corporate concerns; they directly affect patients who could benefit from this novel treatment. Sickle cell disease significantly impacts quality of life for those affected, making timely access to innovative therapies crucial. As Vertex navigates these obstacles, the focus remains on improving patient outcomes while remaining competitive in a rapidly changing environment.
In summary, Vertex’s Casgevy stands at a pivotal point. With ongoing efforts to enhance its rollout strategy, the company must overcome the current challenges to fulfill its promise of offering hope to those suffering from sickle cell disease. The situation underscores the complexities involved in bringing cutting-edge therapies to market, particularly in the face of pressing demand and evolving competition.
-

 Science3 months ago
Science3 months agoALMA Discovers Companion Orbiting Giant Red Star π 1 Gruis
-

 Science3 months ago
Science3 months agoDoctoral Candidate Trivanni Yadav Advances Battery Research at UTulsa
-

 Top Stories4 months ago
Top Stories4 months agoNew ‘Star Trek: Voyager’ Game Demo Released, Players Test Limits
-

 World4 months ago
World4 months agoGlobal Air Forces Ranked by Annual Defense Budgets in 2025
-

 World4 months ago
World4 months agoMass Production of F-35 Fighter Jet Drives Down Costs
-

 Sports3 months ago
Sports3 months agoNASCAR Faces Fan Backlash as Steve Phelps’ Texts Surface
-

 Business4 months ago
Business4 months agoGold Investment Surge: Top Mutual Funds and ETF Alternatives
-

 Top Stories4 months ago
Top Stories4 months agoDirecTV to Launch AI-Driven Ads with User Likenesses in 2026
-

 Politics4 months ago
Politics4 months agoSEVENTEEN’s Mingyu Faces Backlash Over Alcohol Incident at Concert
-

 Science4 months ago
Science4 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Entertainment4 months ago
Entertainment4 months agoFreeport Art Gallery Transforms Waste into Creative Masterpieces
-

 Science4 months ago
Science4 months agoRemembering David E. Brest: A Life Dedicated to Nature and Family









